PLEASE NOTE: We had anticipated a decision after 60 days of the public comments being closed but were informed that it could be up to a year before a decision is made, so CMS has taken no further action on this matter. The current policy (not the new proposed one) should still be in effect.
Proposed changes to Medicare will limit access to treatment with botulinum neurotoxin for spasmodic dysphonia.
If passed, these changes could be adopted by private insurance carriers, which may affect everyone receiving this treatment.
Public comments are accepted until July 13, 2024
PUBLIC COMMENTS ARE NOW CLOSED. THANK YOU TO ALL WHO SUBMITTED.
-
- The proposed guidelines outline specific guidance on dosing, the site of injections, whether they are unilateral or bilateral, and dosing intervals. One example is that the muscle mentioned for the initial injection in all cases is the posterior cricoarytenoid muscle. This muscle is typically only injected in people with abductor spasmodic dysphonia and not adductor spasmodic dysphonia. The muscles that are injected for adductor spasmodic dysphonia are not mentioned anywhere in the guidelines.
-
- The proposed guidelines are not based on commonly accepted practices by the physicians treating these patients nor on current peer-reviewed published articles.
-
- The proposed guidelines place an undue burden on patients and providers and don’t inform clinical care.
-
- Click here to read the proposed LCD, and scroll down to Laryngeal Dysphonia
Fortunately, MACs can’t pass these LCDs without going through a process, which gives us a little time to respond. We reached out to our healthcare professionals, and they are also helping with this issue, including members from the American Academy of Otolaryngology, the American Laryngology Association, the American Broncho-Esophagological Association (ABEA) and the American Speech-Language-Hearing Association (ASHA). These efforts are also supported by member organizations of the Dystonia Advocacy Network.
WE NEED YOUR HELP! Your voice needs to be heard on this issue, especially if these changes will change your access to this treatment. Below is a template of a letter that can be customized and emailed to submit your public comment. The deadline to submit comments is July 13.
TAKE ACTION NOW
-
- Copy and paste the template letter below and add your personal story to voice your concerns about the proposed changes. This is very important.
-
- Find your state below.
-
- Use the email address and subject line for your state. Please note there are two templates depending on the location.
CLICK HERE to open a PDF file with information specific to your state, including the email address and subject line to use. The LCD numbers and email addresses are active links in the PDF.
States with Proposed Local Coverage Determinations for Medicare
Noridian
Alaska, American Samoa, Arizona, California, Guam, Hawaii, Idaho, Nevada, Northern Mariana Islands, Oregon, Washington
policydraft@noridian.com
Public Comment for Proposed LCD – Botulinum Toxin Injections (DL35170)
NGS | National Government Services
Connecticut, Illinois, Maine, Massachusetts, Minnesota, New Hampshire, New York, Rhode Island, Vermont, Wisconsin
NGSDraftLCDComments@anthem.com
Public Comment for Proposed LCD – Botulinum Toxin Injections (DL39832)
Palmetto GBA
Alabama, Georgia, North Carolina, South Carolina, Tennessee, Virginia, West Virginia
A.Policy@PalmettoGBA.com
Public Comment for Proposed LCD – Botulinum Toxin Injections (DL39836)
CGS Administrators
Kentucky, Ohio
cmd.inquiry@cgsadmin.com
Public Comment for Proposed LCD – Botulinum Toxin Injections (DL39857)
WPS | Wisconsin Physicians Service Government Health Administrators
Indiana, Iowa, Kansas, Michigan, Missouri, Nebraska
policycomments@wpsic.com
Public Comment for Proposed LCD – Botulinum Toxin Injections (DL39909)
Template Letter for States with Proposed Local Coverage Determination
Thank you for the opportunity to provide public comment on the proposed changes to the coverage of botulinum neurotoxin (BoNT) injections. I oppose these changes as they will render BoNT useless in providing relief for many people, including those with laryngeal spasmodic dysphonia.
Spasmodic dysphonia is a neurological voice condition that impacts every aspect of a person’s life because the ability to talk is affected. Based on the different forms of this voice condition, there is a great deal of variation in dosing, the site of injections, and unilateral or bilateral dosing intervals. The proposed guidelines are extremely limiting in determining how a physician can provide treatment. They are not based on commonly accepted practices by the physicians treating these patients nor on current peer-reviewed published articles. One example is that the muscle mentioned for the initial injection is never injected for people with adductor spasmodic dysphonia. The muscles that are injected for adductor spasmodic dysphonia are not mentioned anywhere in the guidelines.
As a person with spasmodic dysphonia, I am deeply concerned about these changes and the impact they will have on future treatment options.
[Tell your spasmodic dysphonia story in one paragraph to give your brief history with spasmodic dysphonia and one paragraph to describe how your life would change if this proposed change should become policy]
This proposal, if finalized, would be devastating as it would essentially leave many people with spasmodic dysphonia voiceless, depriving them of access to medically necessary care. So I request that you reject these proposed changes, which will adversely affect our community. Thank you again for this opportunity to share my story.
Sincerely,
Your name
Your address
States without Proposed Local Coverage Determinations for Medicare
Currently, these two MACs do not have a proposed change, but it is on the agenda for the open meeting for public comments in September. Our goal is to prevent these changes from being implemented with the other MACs. However, if the changes do proceed, we want to express our opposition in advance, as the guidelines will likely be the same.
FCSO | First Coast Service Options
Florida, Puerto Rico, U.S. Virgin Islands
ProposedLCDComments@fcso.com
Public Comment regarding potential changes to the LCD on Botulinum Toxin Injections
Novitas Solutions
Arkansas, Colorado, Delaware, District of Columbia, Louisiana, Maryland, Mississippi, New Jersey, New Mexico, Oklahoma, Pennsylvania and Texas
ProposedLCDComments@novitas-solutions.com
Public Comment regarding potential changes to the LCD on Botulinum Toxin Injections
Template Letter for States without Proposed Local Coverage Determination
I am writing regarding proposed changes to the coverage of botulinum neurotoxin (BoNT) injections. Five other Medicare Administrative Contractors have proposed local coverage determinations on this topic (DL39832, DL35170, DL39836, DL39909, DL39857), and I strongly oppose these changes as they will render BoNT useless in providing relief for people with dystonia, including laryngeal spasmodic dysphonia. I understand that you do not have an active draft of a revised LCD on botulinum toxin, but it is listed as a tentative topic for the open meeting in September.
Spasmodic dysphonia is a neurological voice condition that impacts every aspect of a person’s life because the ability to talk is affected. Based on the different forms of this voice condition, there is a great deal of variation in dosing, the site of injections, and unilateral or bilateral dosing intervals. The proposed guidelines are extremely limiting in determining how a physician can provide treatment. They are not based on commonly accepted practices by the physicians treating these patients nor on current peer-reviewed published articles. One example is that the muscle mentioned for the initial injection is never injected for people with adductor spasmodic dysphonia. The muscles that are injected for adductor spasmodic dysphonia are not mentioned anywhere in the guidelines.
As a person with spasmodic dysphonia, I am deeply concerned about these changes and the impact they will have on future treatment options.
[Tell your spasmodic dysphonia story in one paragraph to give your brief history with spasmodic dysphonia and one paragraph to describe how your life would change if this proposed change should become policy]
This proposal, if finalized, would be devastating as it would essentially leave many people with spasmodic dysphonia voiceless, depriving them of access to medically necessary care. If you are considering an update to the current LCD, I request that you reject the proposed changes as presented by the other MACs, as they will adversely affect our community. Thank you again for this opportunity to share my story.
Sincerely,
Your name
Your address
VIDEO | UNDERSTANDING THE ISSUE
PUBLIC COMMENTS
Thank you to the many people who have sent in public comments! People living with spasmodic dysphonia, family, friends, and our dedicated healthcare community. Here are just a few of the public comments.
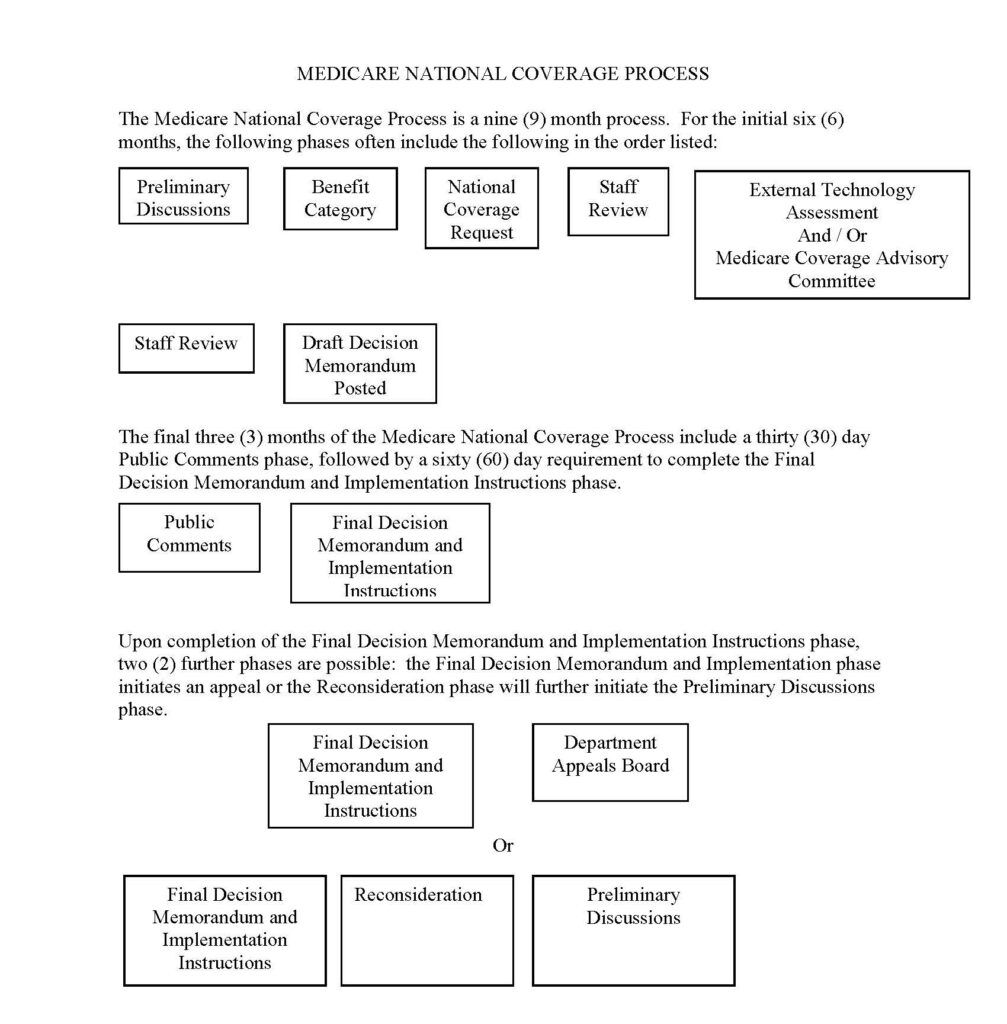
FLOWCHART OF THE NATIONAL COVERAGE PROCESS FOR MEDICARE
After public comments close, there is a sixty (60) day requirement to complete the Final Decision Memorandum and Implementation Instructions phase. Upon completion of the Final Decision Memorandum and Implementation Instructions phase, two (2) further phases are possible: the Final Decision Memorandum and Implementation phase initiates an appeal or the Reconsideration phase will further initiate the Preliminary Discussions phase.